45 fda guidance use of symbols on labels
PDF Symbols Commonly Used in Medical Device and Packaging Labeling Use of the "Rx only" symbol as an alternative to the prescription device labeling statement. FDA Guidance Alternative to Certain Prescription Device Labeling Requirements N/A 2. Alarm to indicate an alarm on a control equipment IEC 60601‐1‐8 Table C1, No. 1 3. Alarm Reset To identify the control for ALARM RESET. Device Labeling | FDA General Device Labeling - 21 CFR Part 801. Use of Symbols - 21 CFR Part 801.15; In Vitro Diagnostic Products ... Final Guidance for Industry and FDA Staff (PDF - 333KB)
PDF Use of Symbols in Labeling - fda.gov use labels with stand-alone symbols in the EU because it is cheaper to a design labeling with a common set of stand-alone symbols versus creating a labeling with no symbols for each EU nation with ...

Fda guidance use of symbols on labels
Recognized Consensus Standards - accessdata.fda.gov Recognized Consensus Standards. This document specifies symbols used to express information supplied for a medical device. This document is applicable to symbols used in a broad spectrum of medical devices, that are available globally and need to meet different regulatory requirements. These symbols can be used on the medical device itself, on ... Use of Certain Symbols in Labeling - Federal Register See FDA guidance entitled "Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use," issued November 30, 2004. Additionally, CDRH has exercised enforcement discretion with respect to the prescription use symbol statement "Rx Only" (without accompanying explanatory text). en.wikipedia.org › wiki › LabelLabel - Wikipedia These labels include information like brand name, class and type designation, and alcohol content. Packaging. Packaging may have labeling attached to or integral with the package. These may carry pricing, barcodes, UPC identification, usage guidance, addresses, advertising, recipes, and so on. They also may be used to help resist or indicate ...
Fda guidance use of symbols on labels. Use of Symbols in Labeling | FDA The Food and Drug Administration (FDA) issued a final rule, Use of Symbols in Labeling, June 15, 2016, that became effective September 13, 2016. The final rule permits the use of symbols in all ... Using Symbols to Convey Information in Medical Device Labeling | FDA The "Use of Symbols in Labeling" final rule which went into effect on September 13, 2016, does not mandate the use of stand-alone symbols in device labeling. Under the final rule, device ... Professional Labeling - Food and Drug Administration Certain OTC monographs explicitly permit professional labeling. When professional labeling is permitted, it can be provided solely to healthcare professionals. In addition, the product itself must be OTC-monograph compliant and should not have the professional labeling or any representations or claims for the professional use directly on the ... FDA Issues Final Rule on Symbols for Device Labels | RAPS The US Food and Drug Administration (FDA) on Wednesday issued a final rule to allow for the use of standalone symbols on medical device and in vitro diagnostic (IVD) labels in an effort to align with international standards. In addition to allowing the use of standalone symbols, the final rule also permits the use of the symbol statements "Rx ...
downloads.regulations.gov › FDA-2013-N-0125-0021Use of Symbols on Labels and in Labeling of In Vitro ... Guidance for Industry and FDA Staff Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use Document issued on: November 30, 2004 The draft of this document was issued on October 28, 2003. The information collection provisions in this guidance have been approved under OMB control number 0910-0553. › blog › selling_handmade_soapRegulations for Selling Handmade Soap & Cosmetics (2021) You must use the genus and species names of the ingredients or the complete INCI name. Please see section 6 for more info. The Cosmetic Regulations has names listed in the Schedule, which are considered “Trivial Names”. You must use the names exactly as they are mentioned in the Schedule of the Cosmetic Regulations. Safety Considerations for Container Labels and Carton Labeling Design ... The collections of information for prescription drug product labeling in § 201.56 and 201.57 (21 CFR 201.56 and 201.57) have been approved under OMB control number 0910-0572. The collections of information described in the FDA guidance for industry entitled "Drug Supply Chain Security Act Implementation: Identification of Suspect Product and ... › media › 85879Guidance for Industry - Food and Drug Administration The use of the word . should. in FDA’s guidance means that something is suggested or ... drug and biological product labels and labeling must 79 comply with FDA regulations in 21 CFR part 201 ...
Safety Considerations for Container Labels and Carton Labeling Design ... This guidance focuses on safety aspects of the application holder's container label and carton labeling design. It provides a set of principles and recommendations for ensuring that critical elements of a product's container label and carton labeling are designed to promote safe dispensing, administration, and use of the product. › business-guidance › resourcesAdvertising FAQ's: A Guide for Small Business | Federal Trade ... Apr 04, 2001 · The FDA handles most matters regarding food labels. For guidance on how the FTC evaluates claims made in food ads, ask the FTC for the Enforcement Policy Statement on Food Advertising. For information about product labeling, visit the FDA's website at or call the FDA Inquiry Line, 1-888-INFO-FDA › medical-devices › device-labelingUse of Symbols in Labeling: Frequently Asked Questions | FDA Yes. The final rule explicitly allows for the optional use of stand-alone symbols in medical device labeling under certain circumstances. We do not expect manufacturers to change existing labeling ... 21 CFR § 801.15 - Medical devices; prominence of required label ... (4) The meaning or explanatory text for the symbol as provided in the FDA recognition or, if FDA has not recognized the standard or portion of the standard in which the symbol is located or the symbol is not used according to the specifications for use of the symbol set forth in FDA's section 514(c) recognition, the explanatory text as provided ...
Summary: Use of Symbols in Labeling (Final Rule) | FDA Summary: Use of Symbols in Labeling (Final Rule) The Food and Drug Administration (FDA or the Agency) is issuing this final rule revising its medical device and certain biological product labeling ...
Where to download free Medical Device Symbols Search on internet. One US FDA guidance document is "Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use", which is freely available for public. You can also find others from the internet search (GHTF, WHO, EU MEDDEV Guidance docs, etc...). But I am not sure of the clarity of the symbols, if you ...
Guidance for Industry and Food and Drug Administration Staff; Use of ... The guidance document entitled "Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use" provides guidance on the use of those recognized symbols. FDA announced the availability of the level 1 draft guidance document in the Federal Register of October 28, 2003 (68 FR 61449). While comments on ...
The FDA's Guidance on Labels and Cartons: Yet Another ... - Finnegan The trade dress of a package "can be considered a protected form of non-verbal communication" and the Guidance (as currently written) unduly restricts freedom of expression by precluding certain information about a business from appearing on the label or labeling. The FDA closed the comment period for the draft Guidance on June 24, 2013 and is ...
Federal Register :: Use of Symbols in Labeling If an FDA-allowed stand-alone symbol is used, for example, in place of the wording "manufacturer:" or "manufacturing site:" followed by a name and address, the final rule requires that a symbols glossary must be included in the labeling for the device to explain the meaning of the symbol to the device's user.
Safety Considerations for Container Labels and Carton ... - fda.gov In this guidance, all such products are jointly referred to as products, drugs, or drug products unless otherwise specified, and persons responsible for designing product container labels and
The FDA considers a "healthy" food label - axios.com Some of the options the FDA is considering for the "healthy" food labels that manufacturers could use. The Food and Drug Administration is testing designs of a label that food manufacturers could voluntarily put on the front of packages indicating that a product is "healthy." Why it matters: The effort is controversial, in part because the ...
Summary: Use of Certain Symbols in Labeling | FDA Analysis of Economic Impacts: Use of Certain Symbols in Labeling: Proposed Rule (PDF - 515KB) Federal Register: 78 FR 23508-23515, April 19, 2013 Docket: FDA-2013-N-0125
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
Use of Symbols in Medical Device Labeling- FDA's Final Rule The final rule provides three options for the use of symbols in device labeling: No use of symbols. Use of symbols with accompanying explanatory text. Use of stand-alone symbols if one of the following conditions is met [2,3]: Scenario a) The symbol is from an FDA-recognized standard and is used in accordance with that standard.
FDA Issues Final Rule on Use of Symbols in Labeling - Lexology Examples of international consensus standard symbols can be found in "Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use," an FDA Guidance ...
eCFR :: 21 CFR Part 801 -- Labeling (4) The meaning or explanatory text for the symbol as provided in the FDA recognition or, if FDA has not recognized the standard or portion of the standard in which the symbol is located or the symbol is not used according to the specifications for use of the symbol set forth in FDA's section 514(c) recognition, the explanatory text as provided ...
PDF Use of Symbols on Medical Device Labels - FDA Requirements MIREGMGR 23rd June 2011 07:07 PM Re: Use of Symbols on Medical Device Labels - FDA Quote: In Reply to Parent Post by adv_webdev (Post 439482) We make medical devices (non-IVD, class 2, some 1) that sell in US as well as in EU and other




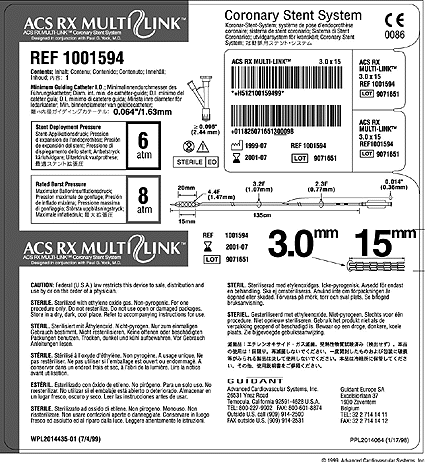
Post a Comment for "45 fda guidance use of symbols on labels"